RABI-767
Revolutionizing care for severe acute pancreatitis
THE PROBLEM
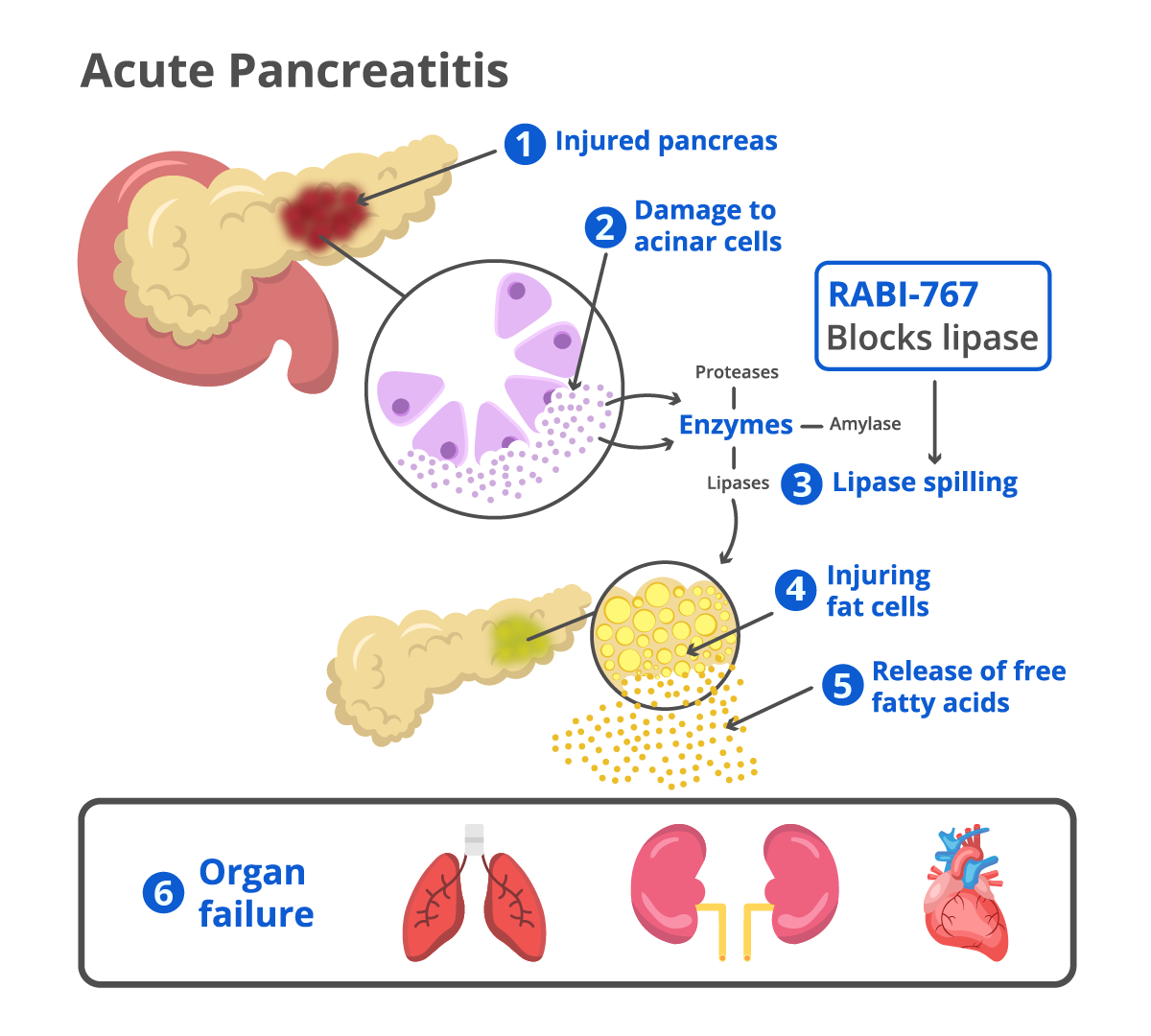
Acute pancreatitis (AP) is an acute inflammation of the pancreas. Gallstones, alcohol, hypertriglyceridemia, and idiopathic acute pancreatitis make up about 70-90% of the etiology of acute pancreatitis [Leppäniemi 2019].
Acute pancreatitis is self-limiting in 70%–80% of cases and does not require any treatment other than parenteral intravenous fluid, analgesics, and supportive care. However, 20-30% of acute pancreatitis patients progress to a more severe form of pancreatitis where the prognosis is far more dire. Patients with severe acute pancreatitis experience prolonged hospitalizations, organ failure and death. There are no approved treatments for acute pancreatitis.

THE COMPOUND
RABI-767 received Fast-Track Designation from FDA in July 2024. RABI-767 is a small molecule pancreatic lipase inhibitor being developed for patients predicted to advance to severe acute pancreatitis. RABI-767 works by halting the highly toxic cascade of fat necrosis, a key driver of tissue injury, systemic toxicity, organ failure and mortality in severe acute pancreatitis.